What are clinical trial phases?
Clinical trials take place in phases. For a treatment to become standard (widely accepted), it must first successfully pass through a series of phases. The early phases make sure the treatment is safe. Later phases show if it works better than the standard treatment. You do not have to take part in all phases.
Phase 1
Purpose:
- To find a safe dose
- To decide how the new treatment should be given
- To see how the new treatment affects the human body and fights cancer
Phase 2
Purpose:
- To determine if the new treatment has an effect on a certain cancer
- To see how the new treatment affects the human body and fights cancer
Phase 3
Purpose:
- To compare the new treatment (or new use of a treatment) with the current standard treatment
There are also very early (phase 0) and later (phase 4) phases of clinical trials, but these trials are less common.
- Phase 0 trials are very small trials that help researchers decide if a new drug should be tested in a phase 1 trial.
- Phase 4 trials look at long-term safety and effectiveness. They take place after a new treatment has been approved and is on the market.
What are the different types of clinical trials?
Clinical trials can typically be categorized into several different types, including:
- Therapeutic trials that enroll patients and provide a specific treatment to the patients to study its impact on cancer.
or
- Non-therapeutic trials that do not provide a treatment to patients, but instead study important factors which help advance the understanding of cancer and its impact.
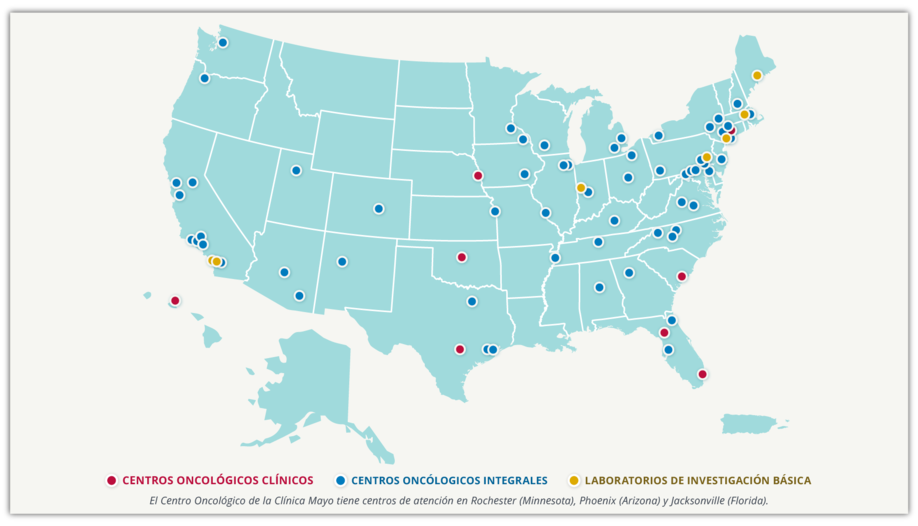
Clinical trials of all types take place all over the country:
The NCI Cancer Centers Program is the backbone of NCI’s effort to study and control cancer. As of June 2023, there are 72 NCI-Designated Cancer Centers, located in 36 states and the District of Columbia. At any given time, hundreds of research studies are under way at the cancer centers, ranging from basic laboratory research to clinical assessments of new treatments
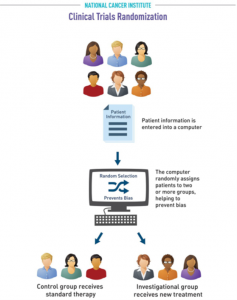
What is randomization?
Randomization is a process used in some clinical trials to prevent bias, which can arise when human choices or other unrelated factors affect the trial’s results. This process helps ensure that unknown factors do not influence the outcomes. If you participate in a trial using randomization, the assignment to either an investigational group (which receives the new treatment being tested) or a control group (which receives the most widely accepted treatment for cancer) occurs by chance. Neither you nor your doctor gets to choose your group.
National Cancer Institute(NCI)
Your assignment is determined with a computer program or table of random numbers.
- If you are assigned to the control group, you will receive the standard treatment for your cancer.
- If you are assigned to the treatment group, you will get the new treatment being tested.
Comparing these groups often clearly shows which treatment is more effective or has fewer side effects. If you are considering joining a randomized clinical trial, you should understand that you have an equal chance of being assigned to either group.
Will I get a placebo?
A placebo is a harmless pill, liquid, or powder with no active effects, often called a “sugar pill.”
Ethical Considerations: It is unethical to give a placebo instead of a treatment that is known to work.
Standard of Care: Clinical trial participants will always be offered at least the standard of care treatment.
Purpose of Placebos in Clinical Trials: Used by researchers to determine if a new treatment is effective by comparing outcomes with those receiving the placebo.
Placebo Use in Cancer Clinical Trials: Rarely used alone in cancer clinical trials unless no known effective treatment exists. Typically, placebos are combined with active drugs in most cancer trials.
Are you eligible for a clinical trial?
Clinical trials have their own guidelines to decide who can take part. Researchers call these “eligibility criteria.” Eligibility criteria can be very specific and each study’s eligibility criteria is different. You can’t be in a study if you don’t match its eligibility criteria. The clinical trial team will let you know if you are eligible.
Standard criteria for entering a trial include:
- Having a particular type or stage of cancer
- Having received (or not having received) a particular type of therapy in the past
- Having specific genetic changes in your tumor
- Being in a particular age group
What it takes to complete a clinical trial?
Listening and learning about clinical trials is a great first step to successfully completing a clinical trial.
After consenting to participate, following the treatment protocol, appointment schedules, and your medication plan closely is key to success. This ensures accurate data for researchers and the best outcomes for you and your community.
Be prepared to share information about your health and communicate with your care team regularly. Not reporting symptoms or missing appoints could deviate results and make them less accurate. If for any reason, you need to reschedule an appointment, need additional support with things like transportation, or even if you fall behind on your medication schedule, contact your clinical care team immediately—they’ll help keep you on track!